February 17th 2025
MagVenture Announces Collaboration with Magnus Medical
Alpharetta, GA, May 1, 2024
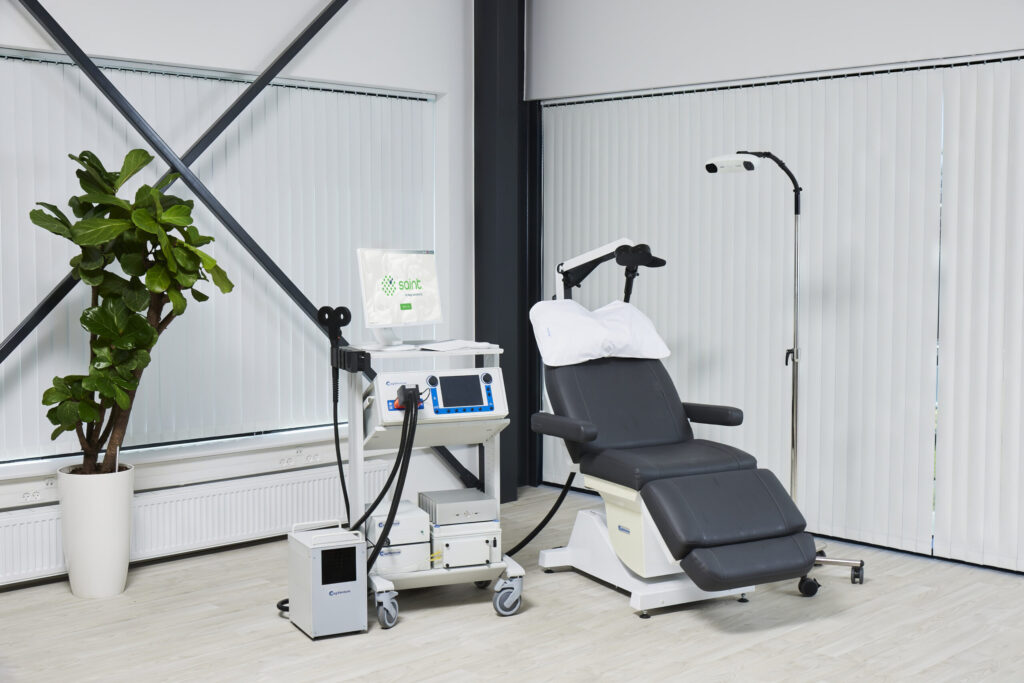
MagVenture is proud to announce its partnership with Magnus Medical, providing its industry-leading MagPro® magnetic stimulation system to support an exciting new chapter in therapeutic transcranial magnetic stimulation (TMS) through Magnus’ SAINT neuromodulation system. SAINT therapy will enable patients to receive a quick, highly effective treatment for Major Depressive Disorder (MDD), especially in the acute setting where TMS has been limited in adoption.
SAINT therapy is an FDA-cleared, rapid-acting, and non-invasive treatment for treatment-resistant major depression in adults who have failed to achieve satisfactory improvement from prior antidepressant medications. Treatment consists of MRI-guided identification of stimulation targets and a proprietary protocol that condenses treatment to just five days.
“MagVenture’s high-performance intermittent theta burst system supports our innovative SAINT therapy, therefore, we are poised to deliver rapid and effective relief to people who suffer from treatment-resistant depression,” said Christian Gormsen, President and CEO of Magnus Medical. “This collaboration underscores our commitment to advancing the treatment of neuropsychiatric disorders and changing people’s lives.”
Kerry Rome, SVP of Sales and Marketing at MagVenture, Inc. continued, “We are excited to enable the expansion of SAINT across the U.S. powered by MagVenture hardware, as well as installation and service support to ensure customers who offer SAINT will experience the same high-quality and reliable operations for which MagVenture is known.”
MagVenture and Magnus Medical will exhibit at the upcoming Clinical TMS Society Annual Meeting in London in June where the SAINT system can be seen firsthand.
About MagVenture
MagVenture is a private, market-leading manufacturer of non-invasive TMS systems worldwide. Headquartered in Denmark, MagVenture has been pioneering leading-edge TMS solutions for more than 30 years. MagVenture’s systems are used for both research and treatment in the fields of psychiatry, neurophysiology, neurology, cognitive neuroscience, and rehabilitation. For more information, visit www.magventure.com
Media inquiries:
Kerry Rome kr@magventure.com or Lauren Schultheiss ls@magventure.com, MagVenture, Inc., +1.888.624.7764
